Super SESI-HRMS: Real-Time Analysis of Dynamic Metabolic Processes
Miriam Macia; Guillermo Vidal-de-Miguel
Abstract
In the last ten years, we have developed the tools required to analyze dynamic changes in metabolism. A key technical challenge was to detect large and biologically relevant molecules in the gas phase, which allowed for the analysis of respiration metabolites. This article describes and illustrates this new window into the metabolism and the volatilome. With an exclusive look at high ionization efficiency of Secondary Electro-Spray Ionization (SESI) and Super SESI application.
Introduction
Whether in good or bad health, the genome of an individual remains, for the most part, the same for its entire life. In contrast, the metabolism is very dynamic and reflects the health status of the individual.
The metabolic responses to nutrients, exercise, disease, drugs, or even the circadian cycles, illustrate how understanding the metabolism often requires capturing the time evolution of the metabolites involved. This applies to living creatures including humans, mice, and cell cultures. Knowing the time evolution of the metabolites and their response to known stimuli is also useful to understand causality relations, i.e. event A triggers metabolite pathways B and C. Our key aim is to provide scientists with instruments that help them detect larger and more biologically relevant metabolites and study their time evolution.
For an analytical instrument to capture these metabolic variations, it must be faster than them. How fast? Hypoxia produces dramatic metabolic changes in a matter of tens of seconds. This perhaps is an extreme, but it serves to define this requirement.
Not triggering a metabolic response with the analytical technique is important to measure the response of the system to the controlled stimuli and not to the instrument. This requires non-invasive analysis. Among the non-invasive biological samples, respiration is an ideal window to the metabolism because it is readily available, and it is constantly produced. Respiration includes the metabolites that diffuse to the headspace of cell cultures, plants, other creatures, and breath. Human breath is particularly easy to collect if the person collaborates. The air surrounding an animal includes its breath and other VOCs, which are more difficult to differentiate. The term volatilome was coined to include all metabolites that can be detected in the gas phase.
Analyzing the volatilome in real-time can be challenging because relevant metabolites are very diluted and tend to have strong carry-over effects. This is because biologically relevant molecules and metabolites of interest tend to be larger molecules with very low vapor pressure. As a result, they are present at minute concentration and tend to condensate on to the walls of the analyzer and the container (i.e. the petri dish and the walls of the incubator in the case of cell cultures).
Detecting very diluted metabolites requires an extremely high sensitivity, down to the ppt and sub-ppt levels. At these concentrations, room air is a very rich and complex mixture, with thousands of species, which requires high separation capacity instruments to separate the different peaks of the spectrum.
High-Resolution Mass Spectrometry HRMS is needed because the chromatographic separation time is not compatible with the short time of the metabolic responses of interest. Interestingly, today's High-Resolution MS provide a combination of separation capacity and sensitivity that should be enough to detect many metabolites of interest well below their vapor pressure. In theory, with Limits of Detection (LoD) in the femtomole range, molecules with vapor pressures in the ppt level should be routinely detected. However, only very light molecules with much higher volatility are normally detected.
To analyze the volatilome in real-time, we have developed a Secondary Electro-Spray Ionization source optimized for Orbitrap HRMS. Here we present how it works, what type of molecules it can detect, and what applications it enables.
Super SESI
Super-SESI uses a water and formic acid nano-electrospray operated near the boiling point of the liquid to produce a cloud of charging ions (protonated water clusters in positive mode and deprotonated water clusters in negative mode), which is mixed with the gas to be analyzed. The neutral molecules in the gas react with the water clusters and get ionized. The ionization mechanism is very soft because the reactions occur at atmospheric pressure and no high energy ions are produced at any point. Because there is virtually no molecule fragmentation, the resulting spectra are very clean and easy to interpret. The ionization efficiency of SESI is very high, as illustrated by several publications [1]. This is partly because, at atmospheric pressure, dilution due to coulombic repulsion is dampened by the gas molecules, and partly because the concentrations of the reagents (the charging ions and the analyte molecule) in the ionizer are very high.
The flow configuration in the Super SESI is optimized to minimize dilution and carry-over effects. For this, the ionizer is operated at high temperature, except near the electrospray, which must be operated slightly below the boiling point of the liquid. In this part of the ionizer, the exposed surfaces are minimized to reduce condensation of low volatility analytes, and the flow configuration is designed to sweep away internal contamination. Click here to learn more information on Super SESI.
Coupled with a regular Orbitrap mass spectrometer, Super SESI opens the possibility for a whole new type of studies. Molecules as large as 700 Da can be detected in real-time in breath and air. In this mass range, and with the high-resolution mass spectrometry, the molecular formula can be easily inferred, easing the interpretation of the results.
What Can Be Detected?
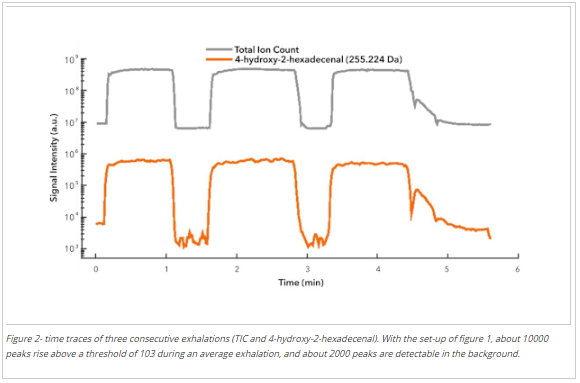
The answer to this question depends greatly on the sample being analyzed. In a study aiming at answering this question, one hundred breath samples of three people were analyzed in real-time with a Super SESI coupled with an Orbitrap Q-Exactive Plus over a period of three months. Figure 2 shows a typical signal profile for three consecutive exhalations. The signal rises during each exhalation and returns to the background level between exhalations. Here we include the Total Ion Count (TIC) and the time trace of 4-hydroxy-2-hexadecenal (whose vapor pressure at 36ºC is 2.7·1 0-7 Bar) to illustrate how low volatility species are easily detected. The average number of peaks above a threshold of 1000 a.u./min was 8000 in the exhalation and 2000 in the background. Most species detected in breath were in the 150 and 300 Da range. The number of species detected declined above 300 Da because many larger molecules with lower volatilities fall below the limit of detection of the instrument. Above 400 Da, the number of species detected in-breath and background overlap because of carryover effects.
A meta-analysis of all SESI related peer-review publications shows that, of all the peaks that can be detected in breath, only about 2.5% have been identified. This shows that there is a lot to be discovered and understood. The number of molecules identified with SESI is low, but it is accelerating, showing that the field is expanding.
Conclusions: Some Key Applications
Therapeutic Drug Monitoring via breath analysis. Currently, therapeutic drug monitoring is based on blood analysis, and it is restricted to a few drugs for which the pharmacokinetic (PK) profile can be described with one or a very few data points. Being non-invasive, breath PK analysis can reach much faster acquisition rates. Thus, enabling more systematic profiling of more drugs. [2]
Breath biomarker discovery. Identifying reliable biomarkers can be challenging in any matrix. Breath can be very complicated because breath samples also reflect the inhaled air, which is an important source of confounding factors. Despite this, several research groups are working looking for biomarkers because a rapid non-invasive breath test would make an ideal screening test for disease. [3]
Bacterial identification: bacterial metabolism produces very distinct smells that can be detected. Understanding the volatilome of bacteria will enable distinguishing different bacterial infections. As antibiotic resistance becomes more prevalent, reducing the time required to identify the bacteria in a bacterial culture will be an important tool to fight infection. [4]
References
[1] Low-Sample Flow Secondary Electrospray Ionization: Improving Vapor Ionization Efficiency; G. Vidal-de-Miguel, M. Macía, P. Pinacho, and J. Blanco; Anal. Chem. 2012, 84, 20, 8475-847
[2] Real-time monitoring of exhaled drugs by mass spectrometry, Christian Berchtold, Marija Bosilkovska, Youssef Daali, Bernhard Walder, and Renato Zenobi; Mass Spectrometry Reviews 2014, 33, 394–413
[3] On-line Analysis of Exhaled Breath: focus review; Tobias Bruderer; Thomas Gaisl; Martin T. Gaugg; Nora Nowak; Bettina Streckenbach; Simona Müller; Alexander Moeller; Malcolm Kohler; Renato Zenobi, Chem. Rev. 2019, 119, 19, 10803-10828
[4] Differentiating Antibiotic-Resistant Staphylococcus aureus Using Secondary Electrospray Ionization Tandem Mass Spectrometry; Haorong Li and Jiangjiang Zhu; Anal. Chem. 2018, 90, 20, 12108-12115
Sources: American Laboratory